Automated Decision Support for Personalized Oncology Treatment Planning in Prostate Cancer (ONCO-TP)
Overview
We are committed to advancing clinical decision support, particularly in oncology treatment planning, with a focus on prostate cancer. In our HSG Health Forward programme-funded ONCO-TP project, in collaboration with Kantonsspital St.Gallen (KSSG), we aim to automate evidence ranking and information extraction to enhance treatment decisions for metastatic castration-resistant prostate cancer (mCRPC) patients. Effective treatment planning in oncology is a complex task that requires integrating diverse and rapidly evolving sources of medical evidence tailored to individual patient profiles. Evidence-based medicine relies on randomized controlled trials (RCTs) as the gold standard, but they are often slow, expensive, and often limited in patient diversity. Biomolecular advancements like next-generation sequencing offer promise for personalized treatment, yet clinical evidence for many tumor-specific features remains sparse. The rapidly expanding oncology literature, growing at 10% annually, makes it increasingly difficult for clinicians to stay updated. Emerging AI technologies, such as large language models (LLMs) like GPT-4, show potential to automate evidence synthesis and extract actionable insights. However, challenges like bias and the need for external validation persist.
The Goal of ONCO-TP
Our objective is to explore the challenges in clinical decision-making and the potential for decision support technologies to transform practice, using a case study in mCRPC. We aim to develop and evaluate innovative automation approaches, including network meta-analysis of aggregate data and individual participant data, to enhance personalized treatment planning. This research will lay the groundwork for further exploring the potential of and creating semi-automated systems that continuously update evidence and apply these methods across different cancer types, integrating clinical and molecular data for broader oncology decision support.
Methods used
- Qualitative mixed method study of field observations and focus group discussions
- Systematic Review and Network Meta-Analysis (NMA)
- Analysis of Individual Participant Data (IPD)
- Development of models to semi-automate evidence synthesis and update datasets as new clinical trial data becomes available.
- Cancer genomics data integration and utilization of molecular and genetic data to enhance personalized treatment planning
Sample results/Publications
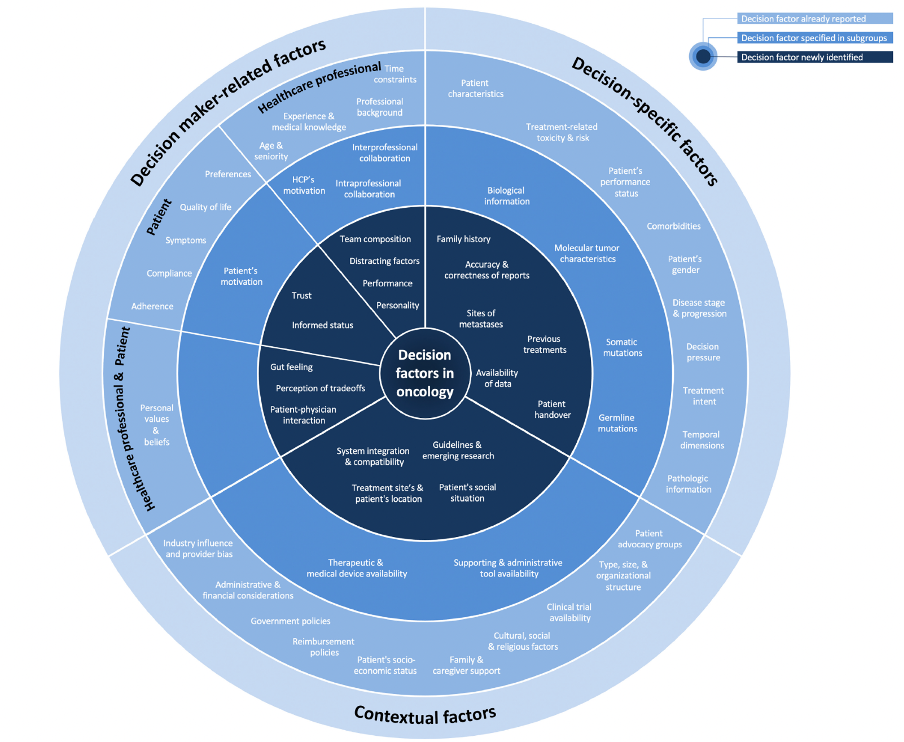
- Factors Guiding Clinical Decision-Making in Genitourinary Oncology
A mixed-methods study was conducted, including field observations and focus group discussions, to assess how treatment decisions are made for genitourinary cancers. We identified 59 factors influencing clinical decision-making, with 23 related to decision-makersm’ personal, cognitive, and emotional attributes, 20 tied to clinical and disease-specific criteria, and 16 contextual factors based on the treatment environment. Our study provides a comprehensive framework for understanding decision-making in genitourinary cancers, highlighting the role of cognitive, emotional, and contextual factors, and emphasizing the need for accessible, high-quality decision-relevant information.
Link to full publication